Abstract
Background: Although the majority of patients with diffuse large B-cell lymphoma (DLBCL) can be cured with intensive chemotherapy and rituximab, 30-40% of patients will be refractory to or relapse after first line treatment. For these patients, the current standard of care is salvage chemotherapy followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT). Prior studies have largely examined clinical risk factors associated with higher risk of relapse after ASCT; however, there is limited data integrating both pathologic and molecular features. Thus, we aimed to identify high-risk features associated with relapse and survival after ASCT using a combination of clinical, molecular, pathologic, and transplant characteristics.
Methods: We retrospectively analyzed the medical records of all adult patients with DLBCL who underwent ASCT at our two institutions from 2010 to 2020. Patients with primary CNS lymphoma, primary mediastinal B-cell lymphoma, or Burkitt lymphoma were excluded. We analyzed demographics, clinical characteristics, cell of origin (COO) by immunohistochemistry (IHC), fluorescence in-situ hybridization (FISH) testing, and treatment/transplant characteristics. The primary endpoints were 3-year progression-free survival (PFS) and overall survival (OS) from ASCT. The Kaplan-Meier method was used to estimate survival with univariate and multivariate Cox proportional hazards regression performed to identify factors associating with PFS and OS, summarized using hazard ratios (HR) with 95% confidence intervals (CI).
Results: A total of 235 DLBCL patients underwent ASCT from 2010 to 2020. Median age at ASCT was 61 years (range: 25-75) and 63% were male. At DLBCL diagnosis, 80% had advanced stage disease, 74% had extranodal involvement, 13% had poor performance status, and 65% had elevated lactate dehydrogenase (LDH). 71 patients (30%) had a prior or concurrent indolent lymphoma diagnosis indicating transformed disease. Of the patients with available COO and molecular data, 115 (60%) had germinal center B-cell (GCB) phenotype by IHC, 10 (6%) had a single MYC rearrangement by FISH, and 35 (22%) had MYC plus BCL2 and/or BCL6 rearrangements (DHL/THL). After first-line treatment, 12% remained refractory and 62% later relapsed at a median of 13 months (range: 1-240). Patients received a median of 2 (range: 1-5) lines of treatment pre-ASCT. At time of ASCT, 66% were in complete response (CR) and 32% were in partial response (PR) by standard of care imaging and response criteria.
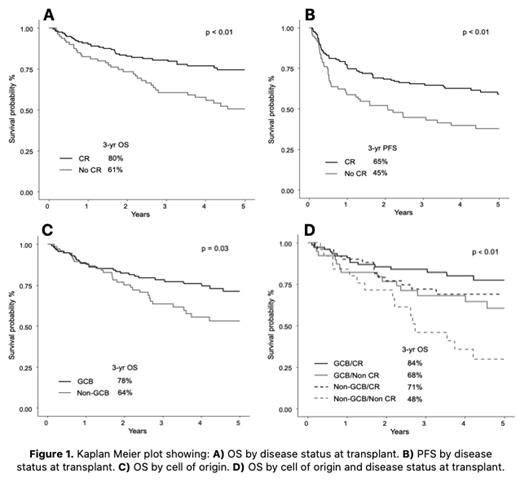
With median follow-up of 5.2 years from time of ASCT, 98 patients (42%) relapsed and 78 (33%) died. 3-year PFS and OS were 58% (95% CI 51-64%) and 74% (95% CI 67-79%), respectively. In univariate analysis, factors associated with worse PFS and worse OS included 3 or more lines of treatment pre-ASCT (p<0.01 for both) and non-CR at ASCT (p<0.01 for both) (Figure 1A and B). Transformed disease was also associated with worse PFS (p=0.03). In multivariate analysis, non-CR at ASCT remained significant (HR 2.22, 95% 1.26-3.90, p<0.01) for worse OS, along with non-GCB COO (HR 1.81, 95% CI 1.03-3.18, p=0.04) and age >60 at ASCT (HR 1.92, 95% CI 1.06-3.47, p=0.03) (Figure 1C). Stratifying by COO and disease status at transplant, 3-year OS was best in the GCB/CR group (84%, 95% CI 73-90%), while worse but similar in the GCB/non-CR and non-GCB/CR groups (68%, 95% CI 51-80% and 71%, 95% CI 56-83%, respectively) (Figure 1D). The non-GCB/non-CR group had the worst 3-year OS (48%, 95% CI 27-67%). No individual factors beyond CR/non-CR at ASCT were associated with worse 3-year PFS. Notably, DHL/THL patients (77% of whom were in CR at time of ASCT) had similar PFS (p=0.08) and OS (p=0.30) to non-DHL/THL patients, suggesting that response to therapy may be more prognostic than high-risk molecular features alone.
Conclusions: This analysis indicated that factors associated with OS after ASCT were disease status at time of transplant and COO, with non-GCB patients not in CR having the poorest outcomes. GCB patients not in CR were still able to be cured by ASCT at a high rate. Molecular rearrangements including DHL/THL were not prognostic, although interpretation may be limited by the modest number of DHL/THL patients. These findings may inform which patients should undergo ASCT, while the highest risk group may be better treated with alternatives including novel targeted agents or chimeric antigen receptor cell therapy.
Bachanova: FATE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; KaryoPharma: Membership on an entity's Board of Directors or advisory committees; Gamida Cell: Membership on an entity's Board of Directors or advisory committees, Research Funding. Fehniger: Compass Therapeutics: Research Funding; Affimed: Research Funding; ImmunityBio: Research Funding; Wugen: Consultancy, Current equity holder in publicly-traded company, Patents & Royalties: related to memory like NK cells, Research Funding; OrcaBio: Other; Kiadis: Other; HCW Biologics: Research Funding; Indapta: Other. Weisdorf: Fate Therapeutics: Research Funding; Incyte: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal